Research
Note – Sharing results of Vascarta’s research, whether completed or in progress, is not intended to provide medical advice nor should it be used as a substitute for the advice provided by your physician or other healthcare provider.
SAFETY
Turmeric (curcumin) is generally recognized as safe by the US Food & Drug Administration as a nutritional product.
A randomized, double-blind, placebo-controlled human safety study of Vascarta’s topical/transdermal curcumin gel, formulated at higher curcumin levels than marketed products, demonstrated that it is well tolerated by healthy subjects, with no adverse effects. This safety trial was performed in 2022 in accordance with ICH E6, Good Clinical Practice.
Conclusion: Overall, Vascarta’s curcumin’s gel formulation demonstrated an excellent safety and tolerability profile. All adverse events were judged UNLIKELY related to the study drug by the investigator. The reported physiological data (blood pressures; brachial artery dilatation) further substantiated the safety of Vascarta’s curcumin formulation at the three (3) doses studied. Specifically, the 24-hour brachial artery dilatation results showed preliminary evidence of enhanced vascular compliance in healthy volunteers.
Data is on file at Vascarta.
ANIMAL RESEARCH
Improved Bioavailability of Vascarta’s topical, transdermal curcumin versus oral curcumin
Topical versus oral administration of curcumin was studied in rats:
- Probal Banerjee, Ph.D., Professor of Chemistry, Biochemistry, and Neuroscience The College of Staten Island (CUNY)
- 2 ml of curcumin administered (oral; topical)
- ~ 8 mg curcumin per dose
- Custom extraction/HPLC protocol specifically designed for curcuminoids
- Pharmacokinetics in both plasma and blood cells
Results
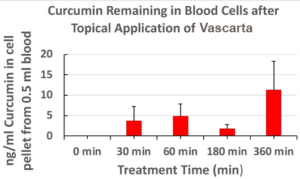
- Orally delivered curcumin – No curcumin detectable in plasma or blood cells
- Topical/transdermal Vascarta curcumin – Curcumin still strongly available in blood cells after 6 hours
Effectiveness Studies in Animals Relating to Directly to Lessening inflammation and Pain Reduction
Studies of Vascarta’s curcumin gel formulation, using rats and mice, have shown effects indicative of lower inflammation and pain including:
Reduced sensitivity to pain and reduced inflammation
- A poster presented at the American Society of Hematology annual conference in December 2023 described work conducted at the University of California at Irvine and other locations. For this work, mice bred to have the painful human sickle cell disease trait, were topically treated either with Vascarta’s curcumin gel formulation or a similar gel without curcumin.
- Within a day, in cold conditions, mice treated with Vascarta’s curcumin gel formulation became less sensitive to painful stimulation (hyperalgesia) compared to the mice not receiving our curcumin formulation, and this effect continued during the full 21-day testing period. In room temperature conditions (mechanical hyperalgesia), the mice experienced less pain starting 14 days after first treatment.
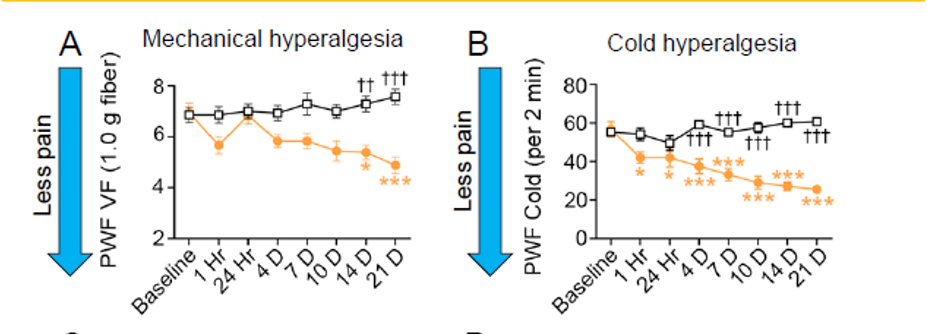
Statistical Significance levels: * P<.05, **,†† P<.01, ***,††† P<.001.
- The study also demonstrated that Vascarta’s topically applied curcumin gel formulation deactivated activated mast cells (MC) and especially granulated mast cells, which are significant indicators of inflammation and contributors to chronic pain.
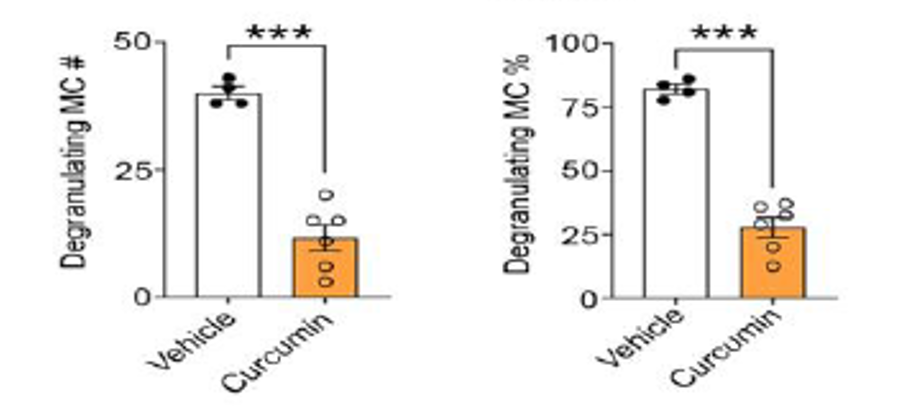
Statistical significance levels: * P<.05,** P<.01, *** P<.001.
- The study demonstrated multiple improvements in markers of inflammation. There was a significant reduction in Plasma SAP (Serum amyloid P-component), the rodent equivalent of C reactive protein for human beings, serving as a marker of systemic inflammation. In addition, as shown below, Vasceptor® significantly reduced inflammatory cytokines including IL-2, IL-4, IL-6, MCP1, INF-γ, GM-CSF, and RANTES.
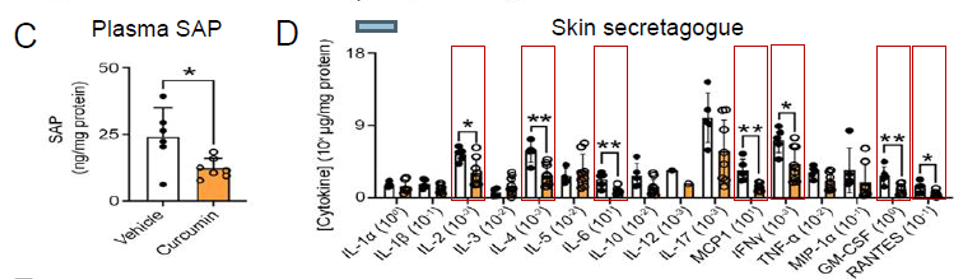
- The study additionally showed clear evidence of enhanced red blood cell stability, decreased hemolysis (red blood cell destruction), enhanced hematocrit, decrease in reticulocytes (immature red blood cells) and a decrease in LDH levels (a marker for hemolysis). Instability of red blood cells is the trigger and promoter of not only the acute and chronic painful episodes in sickle cell disease but also the progression of serious and costly clinical consequences such as cardiovascular disease, renal failure, cognitive decline, sexual dysfunction, slow healing ulcers.
Read published poster: ”Mechanism-based Targeting of Sickle Cell Pathobiology and Pain with Novel Transdermal Curcumin”
Efficacy in Pre-Clinical Endothelial Dysfunction Proof of Concept (Rat Model)
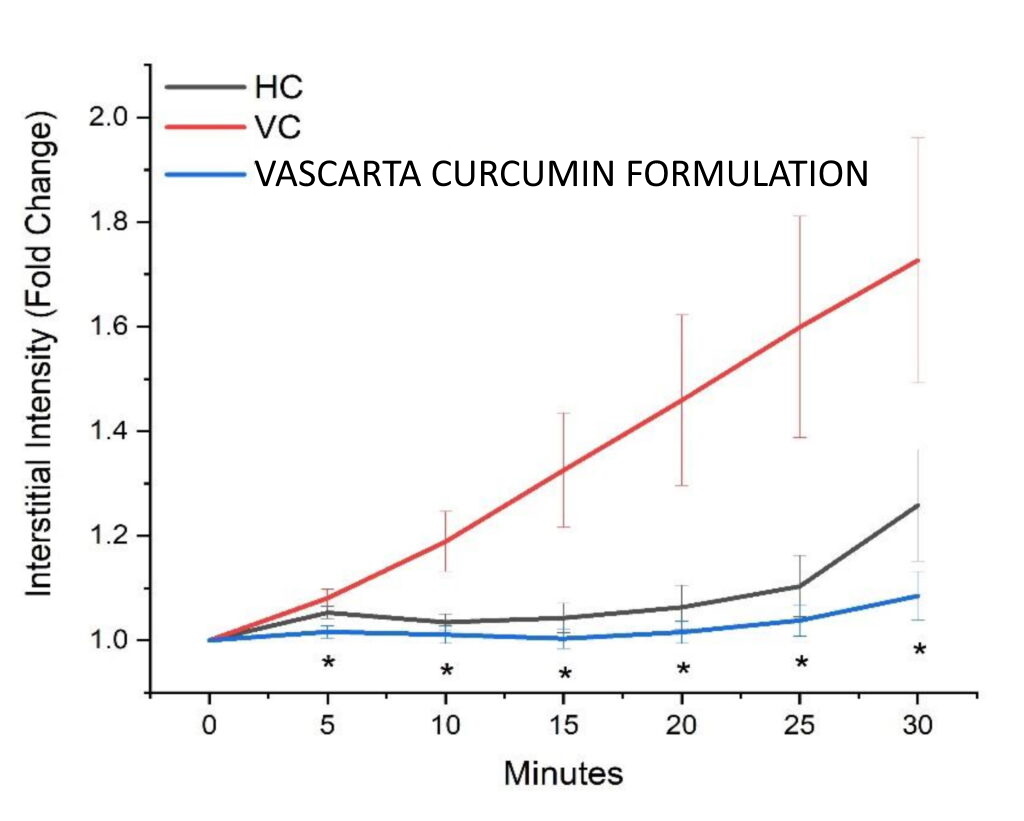
Vascarta’s topical/transdermal curcumin gel formulation prevents vascular leakage (as reflected in the interstitial fluorescence intensity from labeled albumin) that arises from endothelial dysfunction induced from acute lipopolysaccharide-induced (LPS) inflammation.
2021 study – SONG Biotechnologies
HC: healthy control no LPS.
VC: LPS and Vehicle.
Vascarta curcumin gel.
Reduced blood pressure
Efficacy in Pre-Clinical Proof of Concept (Rat Model – Blood Pressure)
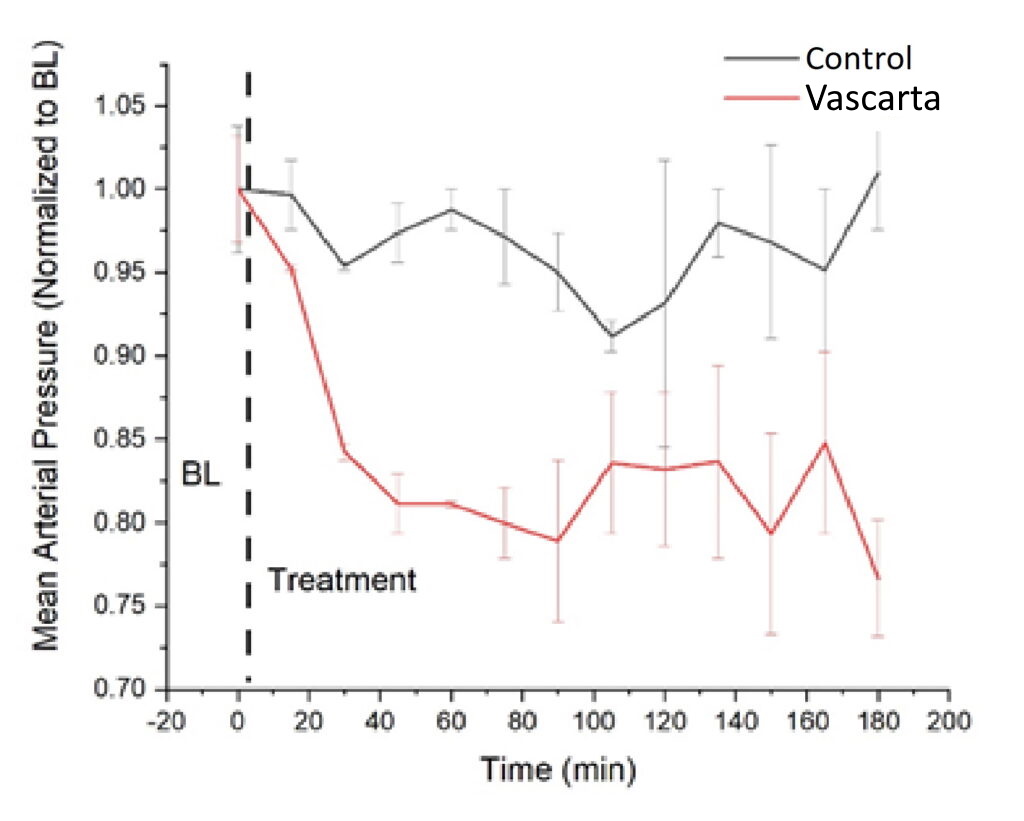
Vascarta’s curcumin gel formulation induces sustained decreases in blood pressure.
2021 study – SONG Biotechnologies
Other Effectiveness Studies in Animals
Although perhaps relating less directly to its anti-pain and anti-inflammation properties, Vascarta’s unique transdermal curcumin formulation has caused researchers to hypothesize numerous additional potential benefits. Current studies underway include those studying these questions:
- Can Vascarta transdermal curcumin improve the quality of donated blood?
- Vascarta has entered into a collaboration with the US Food & Drug Administration (FDA) to study whether the use of transdermal curcumin may improve the quality and duration of stored, donated blood.

- What has been found so far is that, when Vascarta transdermal curcumin is added to human red blood cells starting after 28 days of storage, this results in significant enhancement of the red blood cell profile and extension of refrigerated shelf life.
- As shown below, applying Vascarta transdermal curcumin increases the level of ATP (higher ATP improves blood quality) and decreases the level of protein oxidation (less protein oxidation improves blood quality), along with providing other blood quality benefits.
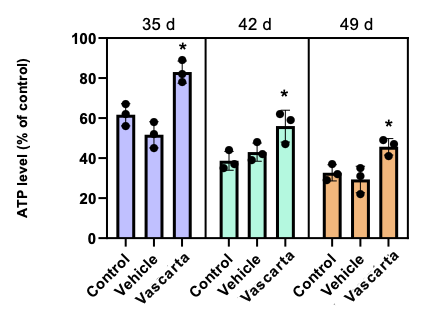
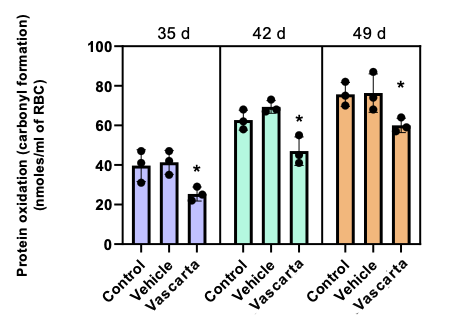
Statistical significance levels: * P<.05
- Because Vascarta transdermal curcumin improves the quality of blood/donated blood which may benefit military personnel with combat trauma, Vascarta is a member of the Medical Technology Enterprise Consortium (MTEC). MTEC is a biomedical technology consortium that focuses on the development of medical solutions that protect, treat, and optimize the health and performance of U.S. military personnel.
2. Can Vascarta transdermal curcumin improve multiple areas of performance and biomarkers with respect to aging?
- Beginning at 20 months of age (equivalent of roughly 55 years old for human beings), male mice were treated twice weekly with a topically applied placebo vehicle gel or Vascarta transdermal curcumin for 3 months. Mice were studied for cognitive effects, physical effects, and biomarker changes.
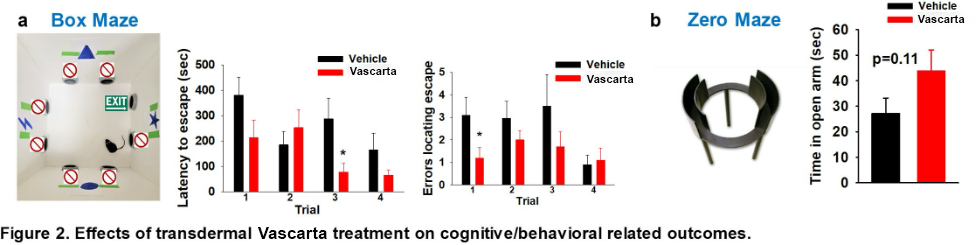
- Cognitive Effects – As they age, mice commonly suffer from reduced memory and increased anxiety. Memory was studied by measuring the amount of time required and the number of errors for the mice to escape from a box maze. Anxiety was studied by measuring the amount of time mice were willing to spend in an open area versus a more secure-feeling high-walled area. Although not statistically significant, there was a trend for mice treated with Vascarta transdermal curcumin to escape the maze more quickly and with fewer errors and to spend more time in the open area.
- Physical Effects – As shown below, in the aged mice treated with Vascarta transdermal curcumin, their weight decreased (a), their balance improved (b), their exercise duration increased (c) and they became less frail overall (d).
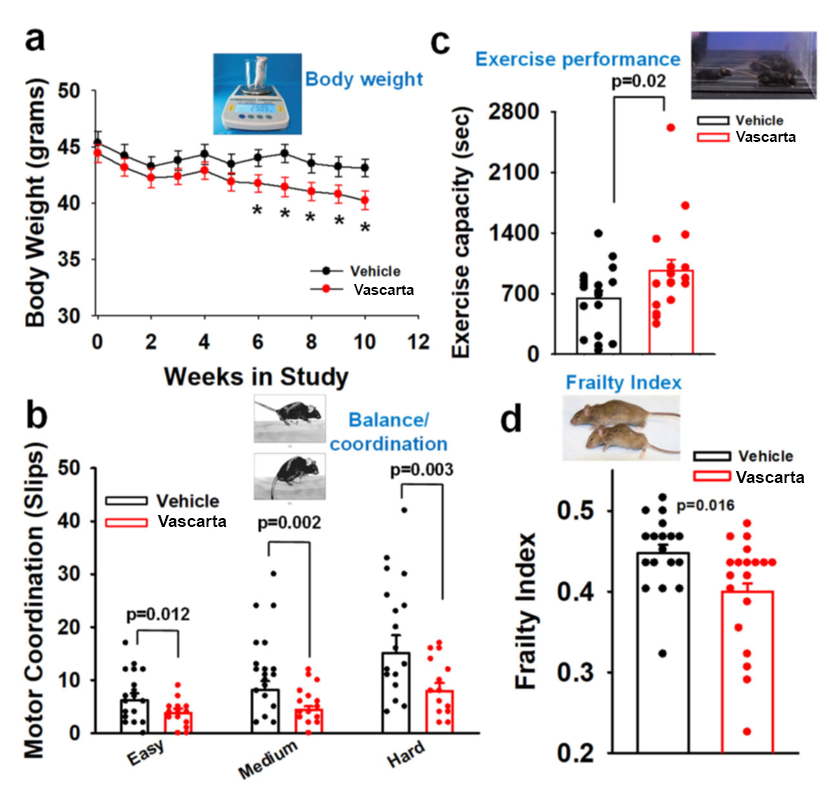
Statistical significance for body weight: * P<.05
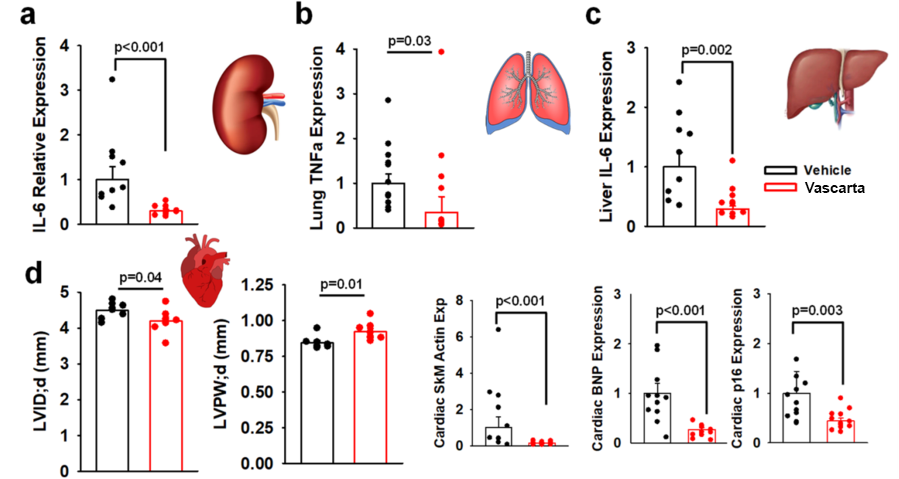
- Biomarker Effects – As shown below, in the aged mice treated with Vascarta transdermal curcumin, expression markers for tissue inflammation, including TNFα (tumor necrosis factor alpha) and IL-6 (interleukin-6), are reduced in multiple tissues including the lung and the liver (a, b, c). In addition, echocardiogram results in the heart suggest Vascarta transdermal curcumin improves cardiac function and lowers markers of cardiac stress and senescence (d).